XL Care Dermic Film IV
Intra venous catheter fixation with integrated non-woven strips
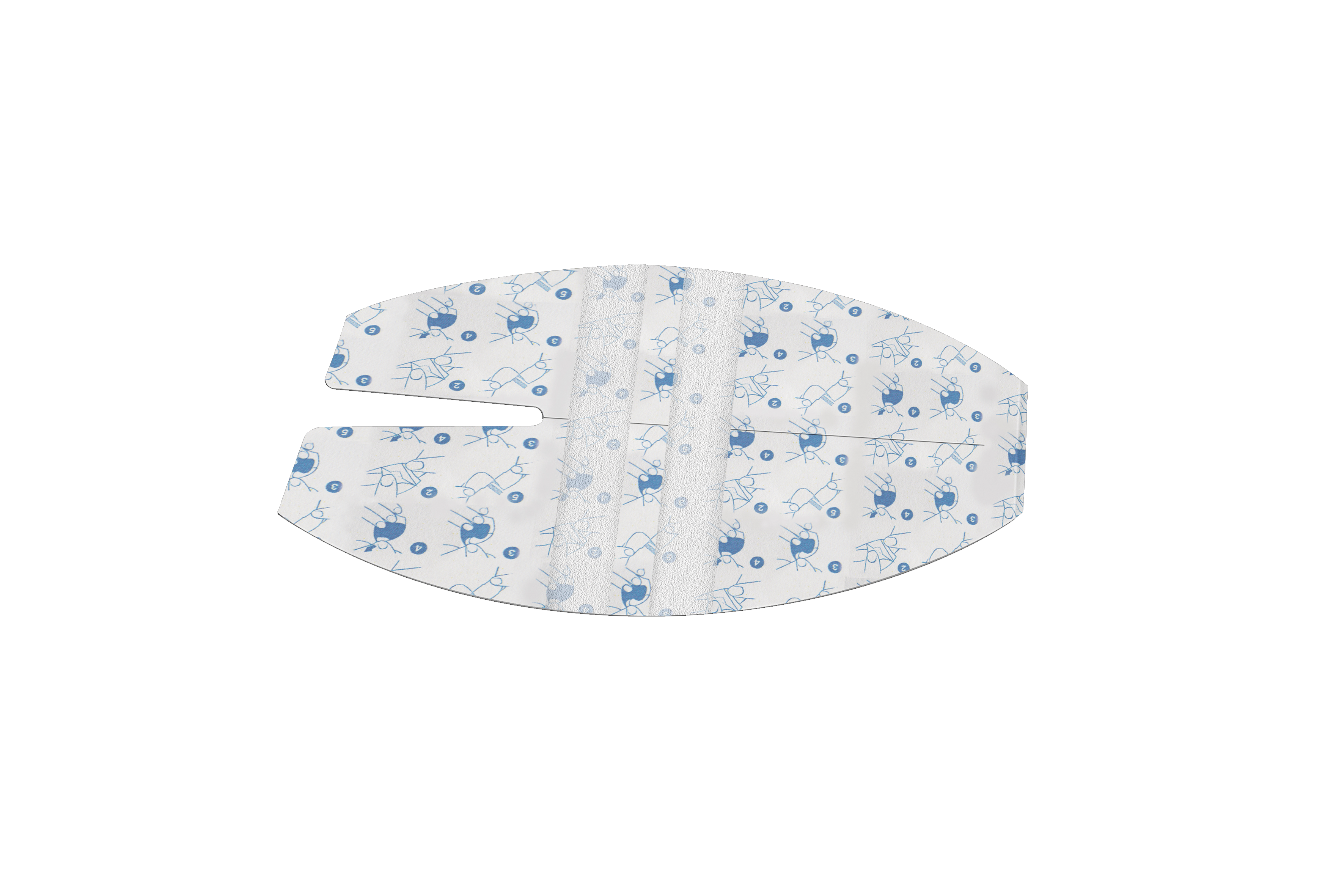
PU Dressing:
- Applicator: Printed white paper one side siliconized 121 g/sqm
- Carrier: Transparent PU film 25 g/sqm
- Adhesive: Solvent acrylic 25 g/sqm
- Liner: Printed paper one side siliconized 81 g/sqm
En savoir plus
→
Dimensions
- Upon request
- Custom printing upon request according to volumes
→
Uses
- Peripheral and central IV catheter securement
- Component of nursing care sets
→
Benefits
- High cutaneous tolerance adhesive: allows the skin to breathe and reduce maceration
- Transparent polyurethane film for puncture site monitoring
- Non-woven reinforcement for extra strength
- Integrated non-woven strips to improve catheter securement
- Backsplit on paper release liner for strips easy delamination
- Breathable, waterproof, bacteria proof and hypoallergenic for safety and protection
- Application tips printed on the paper frame
- Suitable for sterilization, final validation to be performed by the customer
- CE marking
→
Storage and shelf life
- Avoid storing the product outside for any significant period of time where it could be exposed to harmful influences such as humidity or direct sunlight. This product should be transferred, in the original packaging, to the processing area at least 24 hours before
- Product as supplied in original packaging will maintain stated properties during three years before any subsequent transformation / usage / converting, when stored at ambient temperature, preferably in between 15°C (59°F) and 30°C (86°F) and in between 35% and 65% relative humidity.
Check out other similar products
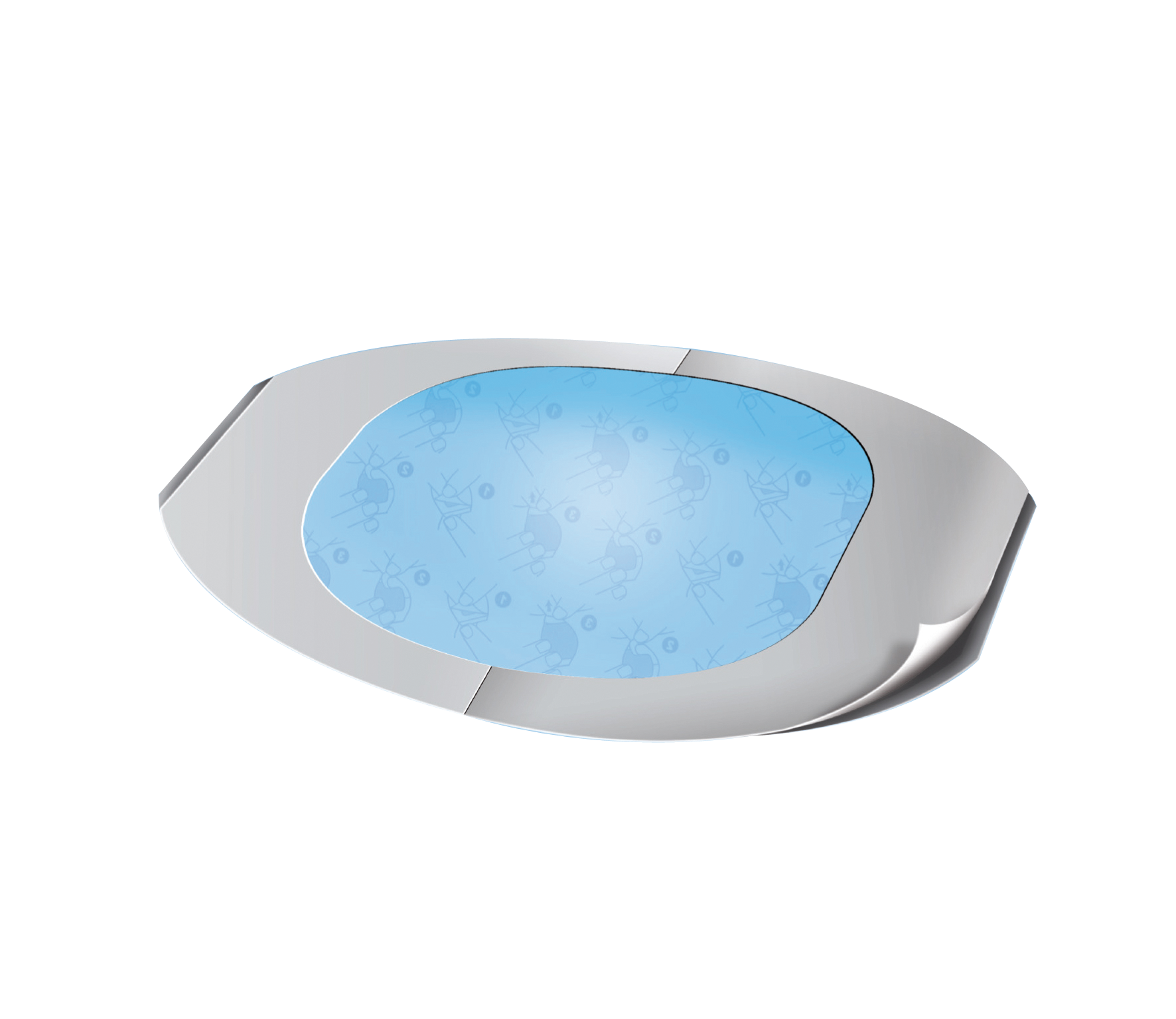
XL Care Dermic Film
Primary device securement
- Applicator: White paper one side siliconized 121 g/sqm
- Carrier: Transparent PU Film 25 g/sqm
- Adhesive: Solvent acrylic 25 g/sqm
- Liner: Printed paper one side siliconized 81 g/sqm
- Transparent adhesive dressing
- Breathable with good skin adhesion
- Application tips printed on the paper frame

XL Care Dermic Film Roll
Transparent PU Film - Wound Protection
- Carrier: Transparent PU film 28 g/sqm with printed PE applicator
- Adhesive: Solvent free acrylic 30 g/sqm
- Liner: Printed paper one side siliconized 63 g/sqm
- High skin tolerance adhesive allows the skin to breath and reduce skin maceration
- Easy to cut to the desired size and shape
- Partly lifted applicator for easy removal by the nurse
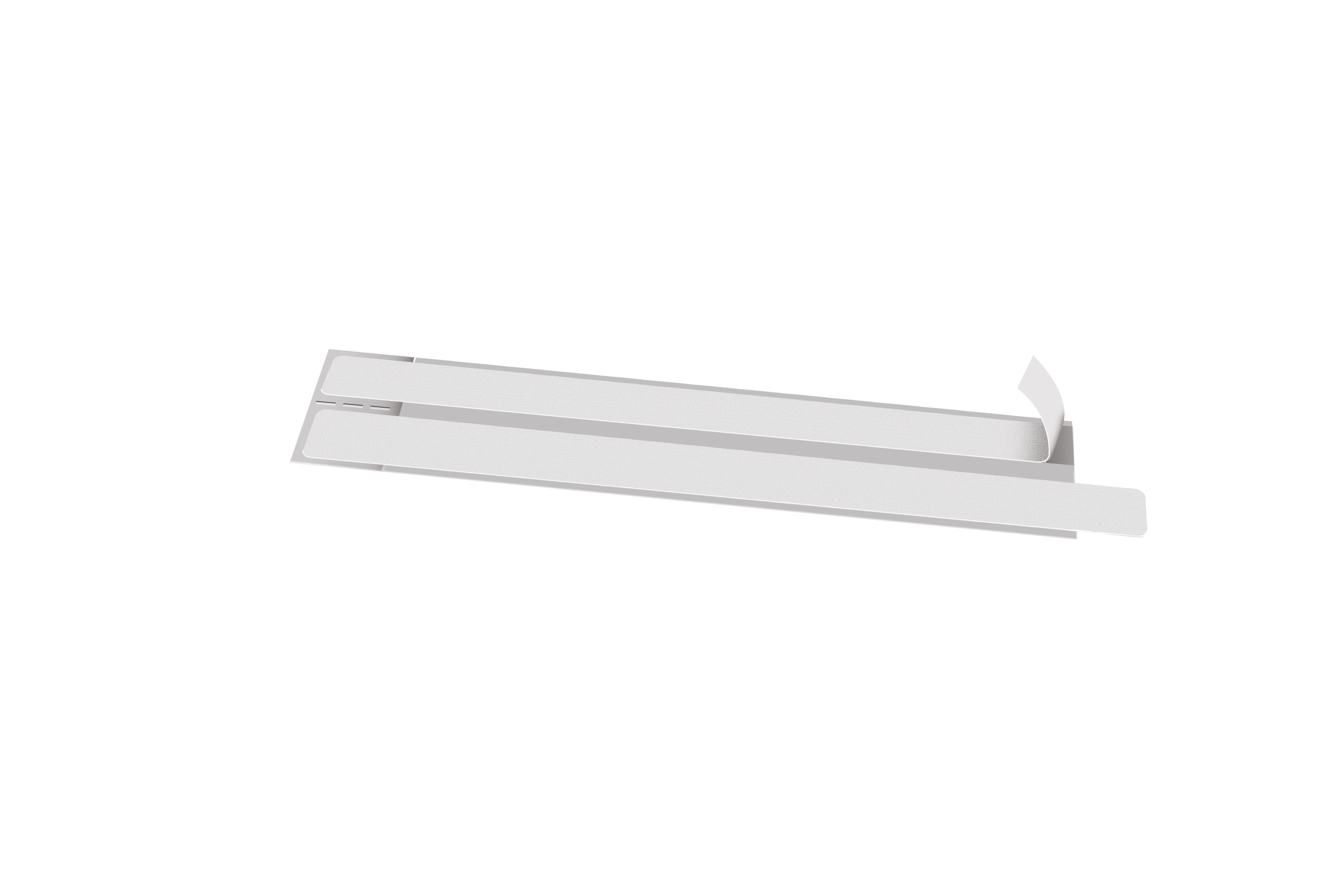
XL Care Dermic Tape
Needle securement - Catheter and gauze fixation
- Carrier: White PET-Cellulose Non-Woven 34 g/sqm
- Adhesive: Water-based acrylic 25 g/sqm
- Liner: White paper, two sides siliconized paper 122 g/sqm
- Skin friendly adhesive to reduce pain at removal
- Microporous white stiff non-woven to ensure air and water vapor permeability
- Individually pre-cut presentation to be applied easily


